Theme: Throwing Light on Latest Techniques for Optimization of Drug Solubility, Delivery and Stability for Maximization of Product Life Cycles
BABE 2017
Track 1: Bioequivalence Studies:
If two medicines are bioequivalent there is no clinically significant difference in their bioavailability. Although bioequivalence is most commonly discussed in relation to generic medicines, it is important to note that bioequivalence studies are also performed for innovator medicines in some situations such as: between early and late clinical trial formulations or between the formulations used in clinical trials and the product to be marketed for new medicines when changes in formulation have occurred after an innovator product has been approved, for example a change in one or more excipients (inactive ingredients).
Related Conferences:
8th Asian Biosimilars Congress August 10-12, 2017 Beijing, China; 10th International Conference and Exhibition on Biologics and Biosimilars Oct 16-17, 2017 Baltimore, US ; 5th International Conference and Exhibition on Pharmacology and Ethnopharmacology Mar 23-25, 2017 Orlando, USA; Annual Bioanalytical Conference Jul 10-13, 2017 Fluno Center, University of Wisconsin-Madison, Madison, WI; 19th International Conference on Pharmacodynamics and Clinical Immunology January 13 - 14, 2017 Zurich, Switzerland.
Related Associations or Societies:
Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry |American Association of Pharmaceutical Scientists.
Track 2: Bioavailability Studies:
Amount of a substance that becomes available (reaches the target organ or systemic circulation) to an organism's body for bioactivity when introduced through ingestion, inhalation, injection, or skin contact. Rate of bioavailability depends on factors such as the type of the substance and the composition of diet.
Related Conferences:
11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry February 27-28,2017 Amsterdam, Netherlands; 3rd International Conference on Biopharmaceutics and Biologic Drugs June 19-20, 2017 Philadelphia, USA; International Conference on Pharmaceutical and Biomedical June 12-13, 2017 Taipei, Taiwan ; International Conference and Exhibition on Pharmaceutical Development and Technology April 24-26, 2017 Dubai, UAE; 2nd Annual Global Drug Bioavailability Enhancement Summit January 24-25,2017 Westin Times Square Hotel-New York, NY.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 3: Factors Affecting Bioavailability:
In pharmacology, bioavailability is a subcategory of absorption and is the fraction of an administered dose of unchanged drug that reaches the systemic circulation, one of the principal pharmacokinetic properties of drugs. There are many recent advances and factors affecting Bioavailability. It includes Absorption, metabolism and Food effect of Drugs. Physico-chemical factors first pass metabolism and Energy dependent efflux transporters are also discussed in BA/BE World Congress.
Related Conferences:
3rd International Conference on Biopharmaceutics and Biologic Drugs June 19-20, 2017 Philadelphia, USA: International Conference on Physical and Theoretical Chemistry September 18-19, 2017 Dublin, Ireland: 2nd International Conference on Bioscience June 19-20, 2017 London, UK: ADVANCES IN DRUG DISCOVERY AND DEVELOPMENT September 20-22,2017 Science City, Kolkata West Bengal, India.
Related Associations or Societies:
European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration.
Track 4: Regulatory Requirements for Bioequivalence :
Bioequivalence regulations have made stricter, yet there is ample scope of improvement in present bioequivalence study designs. Areas where amendments are desired include: general study design, blinding, gender of subject, female subjects, body mass index, and replacement of subjects on withdrawal or, dropouts, genetic phenotyping, endogenous substances, emesis / vomiting and washout period, respectively. Bioavailability and Bioequivalence studies are conducted in healthy human volunteer in study centre. Study centres requires Clinical Pharmacology Unit (CPU) and Bio analytical laboratory. The design and conduct of comparative bioavailability studies are formulated. Investigator(s) should have appropriate expertise, qualifications and competence to undertake a proposed study and is familiar with pharmacokinetic theories underlying bioavailability studies. The design should be based on a reasonable knowledge of the pharmacodynamics and/or the pharmacokinetics of the active substance in question. BA/BE studies are needed by regulations to guarantee remedial proportionality between a pharmaceutically comparable test item and a reference item. BA/BE studies are finished Early and late clinical trial definitions, Formulations utilized as a part of clinical trial and steadiness studies.
Related Conferences:
18th International Conference on Pharmacy and Pharmacology, September 29-30, 2016; International Conference on Drug Formulation, Bioavailability and Pharmacology, Dec 01-02, 2016, Amsterdam; International Conference on Drug Formulation, Bioavailability and Pharmacy, Jan 19-20, 2017, London; 19th International Conference on Ocular Pharmacology and Clinical Pharmacy, January 12-13, 2017, Durban, South Africa; ; International Conference on Drug Formulation, Bioavailability and Pharmacological Sciences, Jan 26-27, 2017, Innsbruck; American Society for Clinical Pharmacology and Therapeutics (ASCPT), March 15-18, 2017, Washington, DC; International Symposium on Drug Delivery and Pharmaceutical Sciences, 09 - 10 March 2017, Kyoto Japan; 8th Global Pharmacovigilance & Drug Safety Summit, April 17-18, 2017 Bali, Indonesia; 8th World Congress on Toxicology and Pharmacology, April 13-15, 2017 Dubai, UAE; International Conference on Pharmaceutical Preformulation and Biopharmaceutics, Jul 04-05, 2017, Singapore; International Conference on Drug Formulation, Bioavailability and Pharmacological Sciences, Nov 13-14, 2017, Tokyo.
Related Associations or Societies:
Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry |American Association of Pharmaceutical Scientists.
Track 5: Bioequivalence Protocols: In vivo/ In vitro studies:
Both bioavailability and bioequivalence focus on the release of a drug substance from its dosage form and subsequent absorption into the systemic circulation. Bioavailability and Bioequivalence studies are required by regulations to ensure therapeutic equivalence between a pharmaceutically equivalent test product and a reference product. Several in vivo and in vitro methods are used to measure product quality.
Related Conferences:
9th Annual Congress on Drug Formulation & Drug Design October 19-21, 2017 Seoul, South Korea; 2nd International Conference and Expo on Generic Drug Market and Contract Manufacturing August 24-25, 2017 Birmingham, UK ; 11th World Drug Delivery Summit October 16-18, 2017 New York, USA; 14th international Conference and Exhibition on Pharmaceutical Formulations Aug 28-29, 2017 Brussels, Belgium.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 6: Study Designs:
It includes randomized, two-period, two-sequence, single dose cross-over design, parallel design and replicate designs. Absolute and Relative bioavailability are discussed. Pharmacokinetics and Pharmacodynamics of the study designs make an important role.
Related Conferences:
5th International Conference and Exhibition on Pharmacology and Ethnopharmacology Mar 23-25,2017 Orlando, USA; Global Meeting on Pharmaceutics & Drug Delivery Systems July 31-Aug 02, 2017 Melbourne, Australia; 9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia; 4th International Conference on Clinical Trials September 11-13, 2017 San Antonio, USA; 9th Annual Congress on Drug Formulation & Drug Design October 19-21, 2017 Seoul, South Korea.
Related Associations or Societies:
European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration.
Track 7: BA/BE studies of Biologics and Biosimilars:
A biosimilar is a biologic therapeutic item which is duplicate of a unique item that is produced by an alternate organization. Biosimilars are authoritatively sanction forms of unique "discoverer" items, and can be produced when the first item's patent terminates. Reference to the pioneer item is a fundamental part of the approbation.
Related Conferences:
7th European Biosimilars Congress May 15-17, 2017 Munich, Germany; 8th Asian Biosimilars Congress August 10-12, 2017 Beijing, China; European Biopharma Congress November 16-17, 2017 Vienna, Austria; World Biosimilar Congress 23-24 May 2017 Hilton San Diego Resort & Spa, San Diego, USA; Biosimilars Global Congress 2017 Europe 25 Sep,2017 - 27 Sep,2017 London ,UK
Related Associations or Societies:
Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry |American Association of Pharmaceutical Scientists.
Track 8: Significance of BA/BE Studies:
This section provides recommendations to the applicants, who undertake bioequivalence studies and/or who wish to request a waiver of in vivo bioequivalence studies for immediate release solid oral dosage forms. Guidance here in explains how the bioequivalence studies should be performed, and when bio waivers can be requested in the context of the WHO Prequalification of Medicines Programme. Data on bioequivalence provide a bridge between two or more pharmaceutical equivalents when safety and efficacy data are available for one of the products, but not for the other. For multisource products bioequivalence studies, conferences are necessary to ensure therapeutic equivalence and interchange ability of the products. Bioequivalence can also be demonstrated by comparative clinical studies, pharmacokinetic studies or appropriate in vitro studies.
Related Conferences:
9th World Congress on BA/BE Studies and Biowaivers July 17-19, 2017 Melbourne, Australia; 11th World Congress on Pharmaceutical Sciences and Innovations in Pharma Industry Feb 27- 28, 2017 Amsterdam, Netherlands; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety July 17-19, 2017 Munich, Germany; International Conference on Biotech Pharmaceuticals October 23-25, 2017 Paris, France;8th Annual Pharmaceutical Analysis Congress Sep 25-27, 2017 Vienna, Austria; 14th International Conference and Exhibition on Pharmaceutical Formulations Aug 28-29, 2017 Brussels, Belgium.
Related Associations or Societies:
European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration.
Track 9: Clinical Trails:
In a clinical trial, participants receive specific interventions according to the research plan or protocol created by the investigators. These interventions may be medical products, such as drugs or devices; procedures; or changes to participants' behaviour, such as diet. Clinical trials may compare a new medical approach to a standard one that is already available, to a placebo that contains no active ingredients, or to no intervention. Some clinical trials compare interventions that are already available to each other. When a new product or approach is being studied, it is not usually known whether it will be helpful, harmful, or no different than available alternatives (including no intervention). The investigators try to determine the safety and efficacy of the intervention by measuring certain outcomes in the participants. Preclinical trials are early experiments performed in the lab, prior to being tested in humans. This early research helps to identify potential treatments that are unsafe or ineffective. Clinical trials used in drug development are sometimes described by phase. These phases are defined by the Food and Drug Administration (FDA).
Related Conferences:
4th International Conference on Clinical Trials September 11-13, 2017 San Antonio, USA; 5th International Conference on October 23-24 Orlando, Florida, USA ;3rd International Conference on Advanced Clinical Research and Clinical Trails September 20-21,2017 Dublin, Ireland ; Paediatrics Clinical Trials Conference February 23-23,2017 Orlando, FL. Oncology Clinical Trials Conference march 20-31,2017 Newport Beach, CA; Clinical Trial Collaborations April 3-4, 2017 Renaissance Boston Waterfront Hotel, Boston, MA..
Related Associations or Societies:
European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration.
Track 10: Clinical Pharmacology and Therapeutics:
Clinical pharmacology is the science of drug use in humans. Clinicians of all specialties pre-scribe drugs on a daily basis, and this is both one of the most useful but also one of the most dangerous activities of our professional lives. This track includes recent developments on behavioural pharmacology, Clinical toxicology, Clinical and experimental pharmacology, Clinical drug developments & therapeutics and also recent developments on posology. Pharmacogenetics is the study of inherited genetic differences in drug metabolic pathways which can affect individual responses to drugs, both in terms of therapeutic effect as well as adverse effects which will be discussed under this track.
Related Conferences:
9th World Congress on pharmacology August 07-09, 2017 Paris France; 8th World Congress on pharmacology and Toxicology July 24-26, 2017 Melbourne, Australia; 10th International Conferences on Immunopharmacology and Immunotoxicology November 20-22 Melbourne, Australia; 10th International Conference on Neuropharmacology and Neuropharmaceuticals Oct 23-24, 2017 Dubai, UAE; American Society for Clinical Pharmacology and Therapeutics March 15-18, 2017 Washington Marriott Wardman Park Washington, DC; 2017 Congress of the French Society of Pharmacology and Therapeutics April 19 - 21, 2017 in Rouen, France; Experimental Biology '17 Annual Meeting of the American Society for Pharmacology and Experimental Therapeutics April 22 - 26, 2017 Chicago, IL, USA.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 11: Advances in BABE:
In pharmacology, bioavailability (BA) is a subcategory of ingestion and is the part of a managed measurement of unaltered medication that achieves the systemic flow, one of the important pharmacokinetic properties of medications. Bioavailability is one of the crucial apparatuses in pharmacokinetics, as bioavailability must be considered when computing doses for non-intravenous courses of organization.
Related Conferences:
17th International Conference and Exhibition on Nanomedicine and Nanotechnology in Health Care Nov 23-24, 2017 Melbourne, Australia; 13th International Conference and Exhibition on Pharmaceutical Nanotechnology July 24-25, 2017 Rome, Italy; 10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems March 13-15, 2017 London, UK; International Conference and Exhibition on Pharmaceutical Development and Technology April 24-26, 2017 Dubai, UAE; 10th Pharmaceutics & Novel Drug Delivery Systems Conference March 13-15, 2017, London, UK.
Related Associations or Societies:
Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology | Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines association.
Track 12: Contract Research Organization:
A contract research organization (CRO) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to governmental organizations. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs that specialize in clinical-trials services can offer their clients the expertise of moving a new drug or device from its conception to FDA/EMA marketing approval, without the drug sponsor having to maintain a staff for these services. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to governmental organizations. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs that specialize in clinical-trials services can offer their clients the expertise of moving a new drug or device from its conception to FDA/EMA marketing approval, without the drug sponsor having to maintain a staff for these services.
Related Conferences:
6th International Summit on GMP, GCP & Quality Control September 25-26, 2017 Chicago, USA; MAGI's Clinical Research Conference - 2017 East May 21-24, 2017 Loews Hotel - Philadelphia, PA; Clinical Trial Supply Europe Conference & Expo 23-25 Jan 2017 Park Plaza Victoria London, London, UK.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 13: Nutrient Bioavailability:
Nutrient Bioavailability refers to the proportion of a nutrient that is absorbed from the diet and used for normal body functions. It includes enhancers of nutrient bioavailability, factors playing a critical role in absorption of nutraceuticals and herbal products. The role of BPDM nutrient bioavailability is discussed under this track.
Related Conferences:
17th International Conference and Exhibition on Nanomedicine and Nanotechnology in Health Care Nov 23-24, 2017 Melbourne, Australia; 13th International Conference and Exhibition on Pharmaceutical Nanotechnology July 24-25, 2017 Rome, Italy ; 10th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems March 13-15, 2017 London, UK; International conference and Exhibition on Pharmaceutical Development and Technology April 24-26, 2017 Dubai, UAE; 10th Pharmaceutics & Novel Drug Delivery Systems Conference March 13-15, 2017, London, UK.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 14: Challenges in Drug Development:
Considerable challenges in drug development, particularly in the face of further organizational changes slated for OGD. A range of legislative and regulatory actions have facilitated consumer access to safe, high-quality generic products, although manufacturing lapses and product quality problems have created critical shortages in important medicines, casting a shadow over the industry's success.
Related Conferences:
11th World Drug Delivery Summit October 16-18, 2017 New York, USA; 10th Conference on Pharmaceutics and Novel Drug Delivery Systems March 13-15, 2017 London UK; Microbiome Drug Development Summit June 27-29, 2017 Boston, MA.
Related Associations or Societies:
European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration.
Track 15: Managing BA/BE Studies:
BA/BE studies are needed by regulations to guarantee remedial proportionality between a pharmaceutically comparable test item and a reference item. BA/BE studies are finished Early and late clinical trial definitions, Formulations utilized as a part of clinical trial and steadiness studies. Everybody has more heaped on their plate than any time in recent remembrance, and numerous consultant discover themselves always re-organizing their work exercises.
BA/BE studies and conferences are required by regulations to ensure therapeutic equivalence between a pharmaceutically equivalent test product and a reference product. BE studies are done for Early and late clinical trial formulations, Formulations used in clinical trial and stability studies, if different Clinical trial formulations and to-be-marketed drug product When it comes to cost and productivity metrics, it’s often said that what gets measured gets done. Part of this is human nature. Everyone has more piled on their plate than ever, and many workers find themselves constantly re-prioritizing their work activities.
Related Conferences:
9th International Conference and Exhibition on Pharmacovigilance & Drug Safety July 17-19, 2017 Munich, Germany; 19th International Conference on Adrenergic Drugs and Adverse Effects Dubai, UAE January 30 - 31, 2017; 9th International Conference and Exhibition on Pharmacovigilance & Drug Safety July 17-19, 2017 Munich, Germany; 2nd International Conference and Exhibition on Marine Drugs & Natural Products June 15-17, 2017 London, UK; 8th Global Pharmacovigilance & Drug Safety Summit July 10-11, 2017 Jakarta, Indonesia.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 16: Assessment of Bioequivalence:
Assessment of the bioequivalence of generic versions of certain reference drugs is complicated by the presence of endogenous levels of said compounds which cannot be distinguished from externally derived compound levels following drug administration. If unaccounted for, the presence of endogenous compound biases towards equivalence in bioequivalence studies of these drugs. Bioequivalence assessments may be complicated further as disposition of the exogenous analogue can be subject to various endogenous processes resulting in nonlinear pharmacokinetics. To overcome these inherent biases a number of different strategies have been employed.
Related Conferences:
9th Annual Congress on Drug Formulation & Drug Design October 19-21, 2017 Seoul, South Korea: 14th International Conference and Exhibition on Pharmaceutical Formulations August 28-29, 2017 Brussels, Belgium: 11th World Drug Delivery Summit October 16-18, 2017 New York, USA: 2nd EUROPEAN CONFERENCE on Pharmaceutics Novel dosage forms & innovative technologies 3 April to 4 April 2017 | Krakow, Poland: 19th International Conference on Respiratory System Diseases March 29 - 30, 2017 Singapore, SG.
Related Associations or Societies:
Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry | American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology | Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines association.
Track 17: Pharma Clinical Trails:
Clinical trials are used to evaluate potential treatments that have had some effect against disease in the lab, or in animal experiments. The whole point of a clinical trial is to find out if a treatment is effective. The aim of clinical trials is to determine if a treatment works and is safe. By comparing similar groups of people taking different treatments for the same disease it is possible to show whether any benefits are due to the treatment. Effective treatments identified in this way may then become standard practice. Since the research is experimental, those who take part in early studies may not always benefit. Once a new approach has been proven safe and effective in a clinical trial, it may become standard practice. Standard practice is a currently accepted and widely used approach and would require approval by a government body such as the FDA or EMEA.
Related Conferences:
4th International Conference on Clinical Trials September 11-13, 2017 San Antonio, USA; 5th International Conference on October 23-24 Orlando, Florida, USA ;3rd International Conference on Advanced Clinical Research and Clinical Trails September 20-21,2017 Dublin, Ireland ; Pediatric Clinical Trials Conference February 23-23,2017 Orlando, FL. Oncology Clinical Trials Conference march 20-31,2017 Newport Beach, CA; Clinical Trial Collaborations April 3-4, 2017 Renaissance Boston Waterfront Hotel, Boston, MA.
Related Associations or Societies:
American Association of Pharmaceutical Scientists | Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry.
Track 18: Challenges in Drug Design:
Any drug that is taken undergoes a number of chemical reactions in the liver as the body attempts to neutralize foreign substances. This set of reactions is well characterized, and a great deal of knowledge exists as to how drugs are modified as the body eliminates them. More importantly, various chemical structures are highly toxic to biological systems, and these are also well characterized. These constraints must also be taken under consideration as novel drugs are developed. The annual decline in the number of drugs approved for release onto the market in recent years has put pressure on you and your team to refine the drug discovery process and use more effective methods to discover a higher number of successful lead compounds.
Related Conferences:
International Congress on Drug Discovery and Development October 19-21, 2017 Phoenix, USA; 4th Annual Congress on Drug Discovery 3rd International Conference and Expo on Drug Discovery & Designing & Designing July 03-05, 2017 Bangkok, Thailand; 9th Annual Congress on Drug Formulation & Drug Design October 19-21, 2017 Seoul, South Korea; 19th International Conference on Drug Design and Pharmacokinetics July 25 - 26, 2017 London, United Kingdom.; ADVANCES IN DRUG DISCOVERY AND DEVELOPMENT September 20-22, 2017 science city, Kolkata West Bengal, India; Drug Discovery and Therapy World Congress July 10-13,2017 Boston, MA,US; 4th NovAliX Conference Biophysics in Drug Discovery June 6-9, 2017 Strasbourg, France.
Related Associations or Societies:
Clinical Trials Information from National Institutes for Health | National Institute of Standards and Technology |Generic Pharmaceutical Association | US Food and Drug Administration European Generic medicines Association | Therapeutics Goods Administration |European Generic medicines Association | Therapeutics Goods Administration | European Economic Area | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry |American Association of Pharmaceutical Scientists.
Track 19: Pharmaceutical Industry: Entrepreneurs Investment Meet:
A platform aimed to connect Entrepreneurs, Proposers and the Investors worldwide. It's intended to create and facilitate the most optimized and viable meeting place for engaging people in global business discussions, evaluation and execution of promising business ideas. An investor could be able to find out the highest potential investment opportunities globally, which provide good return on investment. For entrepreneurs, this would be an ideal place to find out suitable investors and partners to start and/or expand their business. Thus it is a perfect place to connect Entrepreneurs, Business Owners, Early Stage Companies and Established Corporates with National or International Investors, Corporate Investors and Potential Business Partners.


Conference Series invites all the participants across the globe to attend the 8th World Congress on Bioavailability & Bioequivalence: BA/BE Studies Summit June 26-27 2017, at San Diego,California, USA which includes prompt keynote presentations, Oral talks, Poster presentations, Workshops and Exhibitions.
BABE 2017 Conference which is an International Pharma Marketing Industry Conference is a scientific platform to meet fellow key decision makers all-around the Academic Institutions, Healthcare Institutes, Pharmaceutical, Biotech, CROs supply chain, Logistics practitioners making the congress a perfect platform to share experience, foster collaborations through the research talks & presentations to put forward many thought provoking strategies. It's a perfect stage to brainstorm, discover new ideas, search for new skills and a platform to show your capabilities and discoveries to the world. BABE 2017 will be one of the outstanding Bioavailability and Bioequivalence Conferences.The Bioavailability Bioequivalence Research Centre and BA/BE global conferences aims to become a regional center of excellence for assuring the safety and efficacy of generic pharmaceutical products for human use. It plays a key role in the drug development period for both new drug products and their generic equivalents. These studies are also important in the post approval period in the presence of certain manufacturing changes. Information in the overall set of data that ensure the availability of safe and effective medicines to patients and practitioners can be discussed in bioavailability meeting.
Why to attend???
With all the scientific people over the world focused on learning about Pharmaceutical Current and Novel trends and advanced strategies in Pharma Marketing Industry.This is a best globalised opportunity to reach the largest assemblage of participants from the Pharma community. We anticipate participants, renowned speakers and eminent delegates across the globe attending the conference to share their valuable presentation and galvanize the scientific community. BABE 2017 is a 2-day event offering the Exhibition at venue to showcase the new and emerging technologies and Conduct presentations, distribute information, meet with potential scientists, make a splash with new drug developments, and receive fame and recognition. Our services have always met with great achievement in Business Conferencing. World-renowned speakers, the most recent and advanced techniques, developments, and the newest updates are the prominent features of the conference.
Target Audience:
- CRO
- Professors, Associate Professors, Asst Professors
- PhD Scholars
- Graduates and Post Graduates
- Directors, CEO’s of Organizations
- Association, Association presidents and professionals
- Noble laureates in Health Care and Medicine
- Bio instruments Professionals
- Research Institutes and members
- Supply Chain companies
- Manufacturing Companies
Market Analysis of Bioavailability/Bioequivalence:
The pharmaceutical industry is a billion dollar global business empire. According to an OECD (Organization for Economic Cooperation and Development) report, an average OECD country spent 1.5% of its GDP on pharmaceuticals in the year 2015. ‘The rate of growth of pharmaceutical spending surpassed that of total health expenditures in ten of 25 countries, while being roughly equal in six countries. Both pharmaceutical and total health expenditures grew at a higher rate than the mean annual growth rate of GDP for the countries’ that include Ireland, Hungary, United States, Mexico, Australia, Korea, Canada, Slovak Republic, Spain, Luxembourg, OECD, Finland, Iceland, Netherlands, France, Sweden, Portugal, UK, Austria, Czech Republic, New Zealand, Germany, Switzerland, Norway, Denmark, Italy and Japan
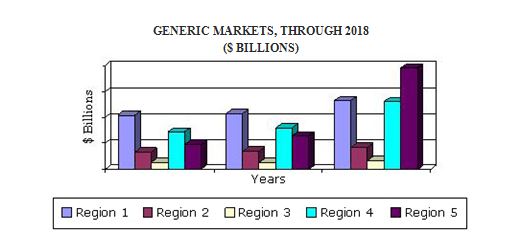
The global generics sector reached $272.8 billion in 2016. This sector is expected to reach $310.9 billion in 2017 and $518.5 billion in 2018, with a compound annual growth rate (CAGR) of 11.5%.
- An overview of the global market for generic drug including coverage of therapeutic
- Analyses of global market trends, with data from 2012, estimates from 2013, and projections of compound annual growth rates (CAGRs) through 2018.The North American market is estimated to reach nearly $79 billion in 2017 and is expected to increase at a 7.9% compound annual growth rate to reach nearly $108 billion in 2020.
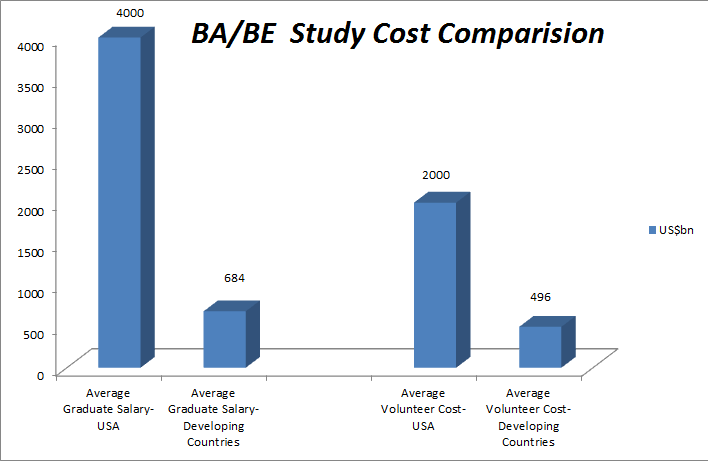
- The United States is currently spending almost $250 billion a year for prescription drugs. If drugs were sold in a competitive market, without government-imposed patent monopolies, this might achieve savings up to $200 billion a year
BABE 2017
We would like to thank all of our wonderful speakers, conference attendees, students, associations and delegates for making BABE 2017 a wonderful event!
8th World Congress on Bioavailability & Bioequivalence: Pharmaceutical R & D Summit hosted by the Conference Series was held during June 26-27, 2017 at San Diego, California, USA at Hilton San Diego Mission Valley with the theme “Throwing Light on Latest Techniques for Optimization of Drug Solubility, Delivery and Stability for Maximization of Product Life Cycles”, which got magnificent response. With the support and guidance of Organizing Committee Members, Editorial Board Members and astonishing presentations of all participants this prominent summit became more impressive.
We received active participation from various scientists, researchers, students and leaders from the field of Pharmaceuticals who made this event successful.
Conference Series would like to convey a great appreciation to Dr. Palmer Taylor & Dr. Pradeep Kakarla as Keynote speakers and Dr. Sudheendra K. for moderating the event.
Keynote Speaker supported this event with sustainable excitement for grand success of this prominent conference. We are expecting to meet the attendees once again at another part of the world for some other pharmaceutical conference.
Conference Highlights
- Clinical Trials
- Pharma Clinical Trials
- Contract Research Organizations
- Challenges in Drug Design
- Challenges in Drug Development
- Bioavailability Studies
- Bioequivalence Studies
- Significance of BA/BE Studies
- Factors Affecting Bioavailability
- Assessment of Bioequivalence
- Study Designs
- Bioequivalence Protocols : In vivo/ In vitro studies
- Managing BA/BE Studies
- Regulatory Requirements for Bioequivalence
- Nutrient Bioavailability
- Advances in BABE
- BA/BE of Biologics & Biosimilars
- Clinical Pharmacology and Therapeutics
- Pharmaceutical Industry: Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | June 26-27, 2017 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioequivalence & Bioavailability
- Journal of Bioanalysis & Biomedicine
- Pharmaceutica Analytica Acta
Abstracts will be provided with Digital Object Identifier by































































