Theme:
BABE 2018
- About Bioavailability & Bioequivalence Conference
- Scientific Sessions/ Tracks
- Market Analysis
- New Updates : Pharmaceutical and Clinical sciences
- Past Conference Report
BABE 2018 CONGRESS
It’s glad to oraganize “9th World Congress on Bioavailability and Bioequivalence” (BABE 2018) in UAE on April 16-18, 2018 at Dubai after a similar series of conferences in consecutive years at USA over the last several years which met with great achievement in Business Conferencing.
Working under the theme "Unfolding Innovations in Bioequivalence/Bioavailability and Related Science" this unique international conference will opportunity to reach the largest assemblage of participants from the Pharmaceutical community to gather and share their insights and convey recent developments in the field of generic drug research and current challenges and possibilities in modelling a new drug and breakthroughs in drug development, Generic drug safety, Novel trends and advanced strategies involving bioavailability bioequivalence research. This is a true forum where ideas and discussion is driven by the participants and interaction with peers and others leads to fruitful outcomes.
BABE 2018 is a 3-day event offering the Exhibition, at venue to showcase the new and emerging technologies and have wider sessions involving Keynote presentation, Oral, YRF ( student presentation), poster, e-poster presentations. World-renowned speakers and eminent delegates across the globe attending the conference, to share their valuable presentation on the most recent and advanced techniques, developments, and the newest updates are the prominent features of the conference.
Target audience:
- CEOs, CROs, Directors, Managers and research associates
- Academic professionals
- Industrial Scientists
- Regulatory and Clinical Scientists
- Researchers, Education providers
- Students and Postdoctoral Fellows
- Pharmacists
- Government Agencies
- Medical Practitioners
- Clinical Pharmacologists
- Clinical Toxicologists
- Molecular and Cellular Pharmacologists
Why to attend??
- Exchange ideas and network with leading pharmaceutical scientists and clinicians and presenting cutting-edge discoveries, research and new therapeutic drugs
- Obtain a global roundup of Pharmaceutical research capabilities and opportunities
Current Research Trends in Bioavailability and Bioequivalence
Track 1: Bioavailability & Bioequivalence
Bioavailability is the measuring of the extent of a therapeutically active medicine that reaches the systemic circulation and is consequently available at the site of action. Bioequivalence is the feature where if two drugs have identical active ingredients contains similar bioavailability and produce the same effect at the place of action.
The measurement of both bioavailability and bioequivalence is crucial to ensure constancy in standards of quality, efficacy &safety of Pharmaceutical dosage forms.
-
BA/BE Studies
-
Bioequivalence Cardiovascular Products
-
Bioequivalence Study Design
-
Bioequivalence Study Protocols
-
Invitro Bioequivalence
-
Universal Bioavailability
-
Bioavailability Metrics
-
Waiver of invivo Bioavailability
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Track 2: Bioavailability Studies and Assessment
Conducting a Bioavailability study enables assessment of the impact of route of administration on BA and defines the absolute bioavailability of the drug released from the drug product. BA for a given formulation provides an assessment of the relative fraction of the orally administered dose that is absorbed into the systemic circulation. If the reference standard is an IV dose, it is referred as Absolute Bioavailability. If the reference standard is any other dosage form than IV, it is referred as Relative Bioavailability. Micro and Micro nutrients play a vital role in bioavailability.
Bioavailability is a subcategory of pharmacological absorption. Bioavailability is generally assessed by finding the area under the plasma concentration–time curve. The factors affecting bioavailability are Pharmaceutics factors, physicochemical properties of drug, Dosage form characteristics & Pharmaceutic Ingredients, Patient related factors like age, Routes of administration (Parenteral, Rectal, Oral, and Topical) etc.
-
Nutrient Bioavailability
-
Absolute Bioavailability
-
Relative Bioavailability
-
Mineral Bioavailability- Micro and Macro
-
Vitamins Bioavailability
-
Bioavailability of Contaminants in Soils & Sediments
-
Factors affecting Bioavailability
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 3: Bioequivalence Studies and Assessment
Bioequivalence studies are done for Early and late clinical trial formulations, Formulations used in clinical trial and stability studies, if different Clinical trial formulations and to-be-marketed drug product When it comes to cost and productivity metrics, it’s often said that what gets measured gets done. Bioequivalence is determined based on the bioavailability of the innovator medicine versus the generic medicine. A typical outline for a bioequivalence study includes organization of the test and reference items on two events to volunteer subjects, with every organization isolated by a washout period. This Study involves parameters on (Cmax) and (AUC), Statistical evaluation.
Assessment of the bioequivalence of generic versions of certain reference drugs is complicated by the presence of endogenous levels of said compounds which cannot be distinguished from externally derived compound levels following drug administration. If unaccounted for, the presence of endogenous compound biases towards equivalence in bioequivalence studies of these drugs. Bioequivalence assessments may be complicated further as disposition of the exogenous analogue can be subject to various endogenous processes resulting in nonlinear pharmacokinetics. To overcome these inherent biases a number of different strategies have been employed.
-
Bioequivalence Protocols: In vitro-In vivo correlation
-
Dissolution Studies
-
Drug-release studies
-
Genetic Phenotyping
-
Response of clinical studies
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 4: Drug Design and development: Challenges
Designing a new drug is a complex, multi-objective problem, demanding the synchronized optimization of target affinity, tissue exposure, formulation, toxicity and so on. Novel drug designers are constantly identifying innovative methods that can be used to improve their drug design. The challenge met nowadays is, how to integrate these multiple inputs and opinions to increase their effect and accelerate drug discovery projects into the clinical outcomes, patient safety for the effective and sustained use of medicines.
-
Computer-Aided Drug Design
-
Rational Drug Design Approach
-
Novel Approach
-
Topical Drug Development
-
Genetics in Drug Development
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 5: Drug Metabolism
Drug metabolism is the term used to describe the biotransformation of pharmaceutical substances in the body so that they can be eliminated more easily. The metabolites of some drugs are pharmacologically active and exert an effect on the body. The active metabolite of some medications is responsible for the principal action of the drug. In this case, the drug formulation is referred to as a prodrug (some chemical substances which do not produce pharmacological effects until they are chemically altered within the body). The rate of drug metabolism affects the efficacy and toxicity of the drug for patients who have very high or low metabolism rates.
- Phase 1 metabolism of drug: P450 (CYP450)Enzyme
- Phase I vs. Phase II Metabolism
- Food/herbal remedies- drug interaction
- Drug Efficacy and toxicity
- Plasma Concentrations and Drug Effects
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 6: Pharmacology- PK & PD approach
Pharmacodynamics and pharmacokinetics are the two principal areas of pharmacology. Pharmacodynamics is the study of the molecular, biochemical, and physiological effects of drugs on cellular systems and their mechanisms of action. Pharmacokinetics focuses rather on how the body affects the drug, in terms of its absorption, metabolism, distribution and elimination. Today, pharmacologists use a variety of techniques, including genetics, molecular biology and chemistry, to explain and manipulate the pharmacological action of substances for health purposes. BA and BE frequently rely on pharmacokinetic measures such AUC to assess extent of systemic exposure and Cmax and Tmax to assess rate of systemic absorption.
It has a broad scope, from the discovery of new target molecules, to the effects of drug usage in whole populations. Clinical pharmacologists work in a variety of settings in academia, industry and government. In the laboratory setting they study biomarkers, pharmacokinetics, drug metabolism and genetics. Bioanalytical method techniques and validation plays a vital role in the evaluation and interpretation of bioequivalence, pharmacokinetics, and toxicokinetic studies. Clinical and experimental pharmacology deals with Clinical drug developments & therapeutics. Pharmacogenomics is the study of how genetic variation influences responses to drugs. This includes how genetic variants affect drug metabolism, efficacy and toxicity, with the goal of improving and personalizing drug therapy.
-
Pharmacokinetics
-
Pharmacodynamics
-
Drug Interactions
-
Drug Safety and Efficacy
-
Posology& Development
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
American Association of Colleges of Pharmacy (AACP), American Pharmacists Association (APhA), American Society for Pharmacy Law American Society of Consultant Pharmacists (ASCP), American Society of Health-System Pharmacists (ASHP), Saudi pharmaceutical society, Pharmaceutical Society of Egypt, Egyptian Society of Pharmacology and Experimental Therapeutics, Iranian Society of Clinical Pharmacist
Track 7: Pharmaceutical Formulations
The general area of study related with the physical, chemical and biological factors influencing the formulation, manufacturing, stability and effectiveness of pharmaceutical dosage forms is called as pharmaceutics. There are different forms into which a drug may be for convenient and effective treatment of disease. Drugs can be prepared for administration by every conceivable route and the suitable preparation is formulated to insure maximum therapeutic response. These may be tablets, capsules, solutions, syrups, elixirs, suspensions, gels, powders, troches or lozenge, ointments, creams, pastes, aerosol, lotions, sprays, inhalants, emulsions and suppositories.
-
Solid Dosage Form
-
Topical Dosage form
-
Parenteral Dosage form
-
Immediate-Release Products
-
Modified-Release Products
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 8: Clinical Research Vs Clinical Trials
Clinical trials are the investigations done in clinical research. Clinical research ecosystem involves a complex network of sites, pharmaceutical companies and academic research institutions. Each clinical trial has a plan of action or a protocol for conducting trial. Clinical trials produce data on safety and efficacy. Different types of clinical research include Treatment, Prevention, Diagnostic, Screening, Quality of life, Genetic studies, Epidemiological studies, Phases of clinical trials (when clinical research is used to evaluate medications and devices)
-
Pre-clinical research/trial
-
Clinical Trial Management
-
Clinical research phase studies
-
In Vitro and In Vivo studies
-
Clinical Study Designs
-
Bioequivalence Protocols: In vitro-In vivo correlation
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 9: Biowaivers: Criteria
Biowaivers are generally provided for multiple strengths after approval of a bioequivalence study. Biowaiver is applied to a regulatory approval process when the application (dossier) is approved based on evidence of equivalence other than an invivo bioequivalence test. For solid oral dosage forms, the evidence of equivalence is determined based on an invitro dissolution profile comparison between the multisource and the comparator product. The purpose of this work was to counsel the biowaivers of biopharmaceutical classification system which are familiar to rise the dissolution, solubility,oral absorption of water insoluble drugs.
-
Waivers of Pharmaceutical Dosage Form
-
Waiver for In vivo bioavailability or bioequivalence
-
Waivers of In Vivo Study Requirements
-
Waiver in Dissolutions
-
Waivers of In Vivo Study Requirements
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 10: BCS & IVIVC Based Biowaivers
The objective of this work was to suggest the biowaivers potential of biopharmaceutical classification system which are known to increase the solubility, dissolution, oral absorption of water insoluble drugs. Biopharmaceutics Classification System and invitro and in vivo classification discusses about ADME pathways of different drugs. This also includes BCS biowaivers, In vitro diffusion cells for dissolution testing in formulation development, In vitro preclinical ADME/BCS testing. Until in vitro in vivo correlation achieves the required degree, the biosimilar drug will not be able to meet the needs of the original drug candidate. The proportion of BCS and IVIVC based biowaivers are low for pharmaceutical products. This classification can be used as a basis for setting in vitro dissolution specifications and can also provide a basis for predicting the likelihood of achieving a successful in vivo-in vitro correlation (IVIVC).
-
BCS biowaivers
-
Preclinical and clinical testing for oral drug delivery
-
In vitro preclinical ADME/BCS testing
-
In vitro drug product research
-
Dissolution testing in drug formulation
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 11: Recent approaches to Biosimilars
The development of biologics calls for overcoming lot many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors and labelling requirements. Also, it is much required now to develop a drug product in accordance to quality by design (QbD). This Bioequivalence conference will look at the multiple facets of current challenges in biosimilar development and focuses on multiple aspects of biosimilar product development to successfully deliver safe, potential and efficacious biologic products to the market.
-
Cancer therapeutics
-
Cardiovascular therapeutics
-
Diabetes therapeutics
-
Analytical strategies
-
Biosimilars: Regulatory approach
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 12: Drug Safety: Pharmacovigilance Scope
Drug Safety is the pharmacological science ensuring safety and related to the collection, detection, assessment, monitoring, and prevention of adverse side effects with pharmacological action of pharmaceutical products. According to US FDA a drug is regarded as safe by looking at side effects, its manufacturing process and results of animal testing and clinical trials. In this track, we discuss Drug safety and its applications in various fields ,Pharmacovigilance and its Significance and Scope present to record its growth and potential as an important discipline within Medical science, and to describe its impact on patient welfare and public health and to know what is pharmacovigilance.
-
Application of drug safety
-
Reporting of ADR
-
Pharmacy Practices and its Challenges
-
Physiological factors affecting drug absorption
-
Regulatory Affairs
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 13: Contract Research Organizations
A contract research organization (CRO) is an organization that provides support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. A CRO may provide such services as biopharmaceutical development, biologic assay development, commercialization, preclinical research, clinical research, clinical trials management, and pharmacovigilance. CROs also support foundations, research institutions, and universities, in addition to governmental organizations. Many CROs specifically provide clinical-study and clinical-trial support for drugs and/or medical devices. CROs that specialize in clinical-trials services can offer their clients the expertise of moving a new drug or device from its conception to FDA/EMA marketing approval, without the drug sponsor having to maintain a staff for these services.
-
Design and analysis of BA/BE studies
-
Statistical evaluation in BA/BE studies
-
Scaling approach for BA/BE studies
-
Bioequivalence trials and clinical endpoint studies
-
Bioequivalence analysis of highly variable drugs
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 14: Regulatory Requirements and Approaches
Bioequivalence regulations have made stricter, yet there is ample scope of improvement in present bioequivalence study designs. Areas where amendments are desired include: general study design, blinding, gender of subject, female subjects, body mass index, and replacement of subjects on withdrawal or, dropouts, genetic phenotyping, endogenous substances, emesis / vomiting and washout period, respectively. Bioavailability and Bioequivalence studies are conducted in healthy human volunteer in study centre. Study centres requires Clinical Pharmacology Unit (CPU) and Bio analytical laboratory. The design and conduct of comparative bioavailability studies are formulated. Investigator(s) should have appropriate expertise, qualifications and competence to undertake a proposed study and is familiar with pharmacokinetic theories underlying bioavailability studies. The design should be based on a reasonable knowledge of the pharmacodynamics and the pharmacokinetics of the active substance in question. BA/BE studies are needed by regulations to guarantee remedial proportionality between a pharmaceutically comparable test item and a reference item. BA/BE studies are finished Early and late clinical trial definitions, Formulations utilized as a part of clinical trial and steadiness studies.
-
WHO Approaches
-
FDA Approach and regulations
-
TGA and risk management approach
-
Food-Effect Bioavailability and Fed Bioequivalence Studies
-
European Guidelines
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Pharmaceutical Chemistry and Drug Design conference, September 3-5, 2018 Dubai, UAE; Molecular Biology and Medicine conference, August 27-28, 2018 Dubai, UAE; Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 3rd Generic Drugs and Biosimilars Conference November 15-17, 2018 Frankfurt, Germany; 12th Pharmaceutical Sciences and Innovations in Pharma Industry Congress February 26- 27, 2018 London, UK; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; Dubai International Pharmaceuticals and Technologies Conference February 27-March 1 Dubai, UAE; Global Pharma Meet April 02-04, 2018, Dubai; Pharmaceutical & Pharmacological Research Conference, March 28-29, 2018 Dubai, UAE; Global Pharma Meet & Expo February 26-28, 2018 Dubai, UAE; Pharmaceutics & Novel Drug Delivery Systems conference , April 09-11, 2018 Boston, USA
Related Associations or Societies:
Generic Pharmaceutical Association , US Food and Drug Administration( FDA) , European Generic medicines Association , European Economic Area , Canadian Generic Pharmaceutical Association (CGPA) , Bioequivalence and Bioavailability forum , American Association for Clinical Chemistry (AACC) , American Association of Pharmaceutical Scientists (AAPS) , National Institutes for Health (NIH) , National Institute of Standards and Technology (NIST)
Track 15: Recent advancements in BA/BE Research
The aim of bioavailability study is to find out the dosage form influence on the biological performance of the drug, sensitivity to detect differences in the rate and extent of absorption. Bioavailability and bioequivalence study design involves Single dose or multi dose standard 2x 2 crossovers, Parallel groups, for more than two formulations. A study design meant for estimating essential pharmacokinetic parameters differs significantly from a bioequivalence study meant for comparing the test formulation. The results of a pilot study can be used as the sole basis to document BA or BE provided the study’s design and execution are suitable and enough subjects have completed the study.
-
Novel Drug Delivery Systems- BA/BE approach
-
Generic drugs: Current claims and future directions
-
BA/BE Studies for Immediate-Release Solid Oral Dosage Forms
-
Bioequivalence analysis of highly variable drugs
-
Bioavailability Study for cancer drugs
-
Food-effect Bioavailability and fed Bioequivalence studies
Related Pharmaceutical Research Conferences | Pharmaceutical Sciences Meetings | Generic Drug Events | Clinical Research Conferences
Advanced Materials Science & Nano Technology conference ,August 27-29, 2018 Dubai, UAE; 5th Chemistry in Drug Discovery & Designing congress April 16-17, 2018 Dubai, UAE; Medicinal and Pharmaceutical Chemistry conference July 19-21, 2018 Dubai, UAE; 16th Pharmaceutics & Novel Drug Delivery Systems Conference March 19-21, 2018 Berlin, Germany; 15th European Pharma Congress, May 07-09, 2018 Frankfurt, Germany; 4th Drug Discovery, Designing and Development Conference June 27-28, 2018 Vancouver, Canada ; Pharmaceutical conference and expo March 21-23, 2018 Philadelphia, USA; Pharma Conference and Expo May 2-4, 2018 | Rome, Italy.; 11th Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology Meeting March 19- 22 2018 Granada, Spain; 20th Pharmacy and Pharmaceutical Sciences Conference February 15 - 16, 2018 London, United Kingdom; 11th Pharmacovigilance Conference 24 January 2018 London ,UK
Track 16: Pharmaceutical Industry: Entrepreneurs Investment Meet
A platform aimed to connect Entrepreneurs, Proposers and the Investors worldwide. It's intended to create and facilitate the most optimized and viable meeting place for engaging people in global business discussions, evaluation and execution of promising business ideas. An investor could be able to find out the highest potential investment opportunities globally, which provide good return on investment. For entrepreneurs, this would be an ideal place to find out suitable investors and partners to start and/or expand their business. Thus, it is a perfect place to connect Entrepreneurs, Business Owners, Early Stage Companies and Established Corporates with National or International Investors, Corporate Investors and Potential Business Partners.
Related Associations or Societies:
USA: American Association of Colleges of Pharmacy (AACP), American Pharmacists Association (APhA), American Society for Pharmacy Law American Society of Consultant Pharmacists (ASCP), American Society of Health-System Pharmacists (ASHP), American Association of Pharmaceutical Scientists(AAPS), American College of Clinical Pharmacy(ACCP), Accreditation Council for Pharmacy Education(ACPE), Academy of Managed Care Pharmacy(AMCP), American Society of Health-System Pharmacists(ASHP) Alpha Zeta Omega(AZO), Connecticut Pharmacists Association(CPA), International Society for Pharmacoeconomics and Outcomes Research(ISPOR), National Community Pharmacists Association(NCPA), National Pharmaceutical Association(NPhA), Parenteral Drug Association (PDA), Phi Lambda Sigma Society(PLS), Rho Chi Society, Student National Pharmacy Association (SNPhA), Pharmaceutical Manufacturers Association of Canada (PMAC), American Association of Pharmacy Technicians (AAPT).
Europe: Norwegian Pharmacy Association. European Association of Employed Community Pharmacists in Europe (EPhEU),European Pharmaceutical Union (EPU) ,Pharmaceutical Group of the European Union (PGEU), National Pharmacy Association, Pharmaceutical Society of Northern Ireland, Royal Pharmaceutical Society (RPS), Danish Association of Pharmaconomists, German Pharmaceutical Society, The French Pharmaceutical Companies Association, Spanish Society of Hospital Pharmacists, Italian Pharmaceutical Association, Spanish Association of Pharmaceutical Industry, The Association of the British Pharmaceutical Industry, Royal Pharmaceutical Society (RPS), The National Pharmacy Association (NPA), British Generic Manufacturers Association, Swiss Association of Pharmaceutical Professionals, Hellenic Association of Pharmaceutical Companies, Royal Dutch Pharmaceutical Students'​ Association (KNPSV), Polish Pharmaceutical Students' Association
Middle-East: Saudi pharmaceutical society, Pharmaceutical Society of Egypt, Egyptian Society of Pharmacology and Experimental Therapeutics, Iranian Society of Clinical Pharmacist, Pharmaceutical Association of Israel, Iranian Association of Pharmaceutical Scientists (IASP), Pharmaceutical Manufacturers Association of Turkey, Iraq Pharmaceutical Association, Lebanese Pharmaceuticals Importers Association, Jordan Pharmaceutical Association, Jordanian Association of Pharmaceutical Manufacturers, Oman Pharmaceutical Society
Asia-Pacific: Australian College of Pharmacy, Pharmaceutical Society of Australia, The Pharmacy Guild of Australia, The Society of Hospital Pharmacists of Australia, Chinese Pharmaceutical Association, Indian Pharmacist Association, Korean Pharmaceutical Association (KPA), Indian Pharmacological Society, Japan Pharmaceutical Manufacturers Association(JPMA), Japan Pharmaceutical Association, Vietnamese Pharmaceutical Association, Vietnam Pharmaceutical Companies Association, Pharmaceutical Society of Singapore, (SAPI)Singapore Association of Pharmaceutical Industries, Thai Pharmaceutical Manufacturers Association, Pharmaceutical Research & Manufacturers Association (PReMA), Pharmaceutical Association of Thailand, The Pharmaceutical Society of Hong Kong, Pakistan Pharmacist Association
Market Analysis of Pharmaceutical Industry in emerging Genric drugs:
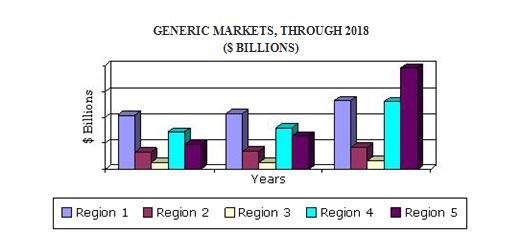
The global generic drugs market 2016 report forecasts patent expiry of drugs worth $150 billion by 2020, which a key growth driver for top-selling drugs as well as new, inexpensive generic drugs. With many pharmaceutical drugs set to lose their patents during the forecast period, the competition in the generic market is expected to increase. Patent expiry of a branded drug results in the introduction of inexpensive generic drugs in the market. Branded drugs with sales of up to $135 billion expired by the end of 2015, offering pharmaceutical companies opportunities to capitalize on this market.
The analysts forecast global generic drugs market to expand at a compound annual growth rate (CAGR) of 10.53% during the period 2017-2020. One trend which is influencing generic drugs market growth is the outsourcing of drug development. Some vendors outsource parts of their R&D process, such as product characterization testing, and toxicology testing, to contract research organizations. Similarly, some of them outsource parts or even the entirety of their manufacturing processes to other organizations. In this way, outsourcing is becoming a trend in the global generic drugs market.
Major Pharmaceutical Associations around the Globe
- American Association of Pharmaceutical Scientists (AAPS)
- International Pharmaceutical Federation (FIP)
- International Pharmaceutical Students' Federation (IPSF)
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- European Generics and Biosimilar Medicines Association (EGA)
- Canadian Generic Pharmaceutical Association (CGPA)
- Generic Pharmaceutical Association (GPhA)
- ORPHANET Parenteral Drug Association
- Association of the British Pharmaceutical Industry (ABPI)
Top universities in UAE:
- Gulf Medical University
- University of Sharjah
- Dubai Pharmacy College
- Ajman University
- Ras al-Khaimah Medical and Health Sciences University
- Al Ain University of Science and Technology
New Clinical study on Molgradex to treat NTM lung infection
Savara Initiates Phase 2a Clinical Study of Molgradex for the Treatment of NTM Lung Infection
As per Savara Inc. a vagrant lung illness organization, declared the start of a Phase 2a clinical investigation, OPTIMA, assessing its lead item applicant Molgradex, a breathed in definition of recombinant human granulocyte-macrophage state invigorating component (GM-CSF), for the treatment of nontuberculous mycobacterial (NTM) lung disease. Molgradex is additionally right now being examined in a worldwide critical Phase 3 clinical examination, IMPALA, for the treatment of immune system aspiratory alveolar proteinosis (PAP).It has been allowed Orphan Drug Designation for the treatment of PAP in the United States and the European Union.
NTM lung disease is an extensive remedial test because of the interesting capacity of these microorganisms to sidestep the ordinary executing instruments of alveolar macrophages, a sort of insusceptible cell in charge of guarding against microbes in the lungs. Logical research in different creature models, including GM-CSF knockout mice, have shown that GM-CSF assumes a vital part as an effector particle enacting macrophages to eliminate mycobacteria, with or without the synchronous utilization of anti-toxins. Among the different NTM species, Mycobacterium abscesses (M. abscesses) is an especially difficult clinical issue, being a standout amongst the most safe life forms to anti-infection agents.
"Treatment of NTM lung contamination with long multi-tranquilize anti-microbial regimens is testing, places critical weight on patients, but every now and again neglects to annihilate the disease," expressed Rachel Thomson, M.B.B.S., Ph.D., Thoracic Physician, Associate Professor, The University of Queensland, Australia and one of the planning specialists on the OPTIMA examine. "In light of the developing logical method of reasoning and the empowering results of the main clinical cases treated with breathed in GM-CSF, I trust Molgradex speaks to a promising novel approach into this ailment urgent for more compelling treatment alternatives."
About the OPTIMA Phase 2a Clinical Study
OPTIMA is an open-name, non-controlled, multi-focus, Phase 2a clinical investigation of Molgradex in 30 subjects (≥18 years old) with persevering pneumonic NTM contamination. OPTIMA will enlist subjects with ceaseless M. abscessus or Mycobacterium avium complex (MAC) contamination, with all subjects having either anti-infection obstinate disease or bigotry to standard NTM anti-toxins. Subjects with CF won't be selected.
About NTM Lung Infection
NTM lung disease is an uncommon and genuine lung issue related with expanded rates of bleakness and mortality. Nontuberculous mycobacteria are normally happening life forms and NTM lung contamination can happen when an individual breathes in the living being from their condition and builds up a gradually dynamic and dangerous lung infection.
New research hopes to decrease symptoms in regularly utilized Medications
New analysis from The Australian National University (ANU) has bored right down to the sub-atomic level to find similarities crosswise over six pharmaceutical medications used as a section of facilitate with discomfort, dental specialist sedative, and treatment of brain disorder, in a proposal to work out a way to decrease undesirable reactions. One out of 5 Australians encounter endless torment, and 250,000 Australians suffer brain disease, 40 % of that are children.
As of recently, specialists have realized that medications which treat torment and epilepsy are successful, some of which have even been utilized clinically since the 1950s. Yet, atomic points of interest of how they function and why they cause symptoms have not been considered until moderately as of late, because of new innovation.
Dr Amanda Buyan from the ANU Research School of Biology expressed profound gratitude to the National Computational Infrastructure (NCI) supercomputing power at ANU, analysts are presently ready to run greater and more perplexing reenactments to show signs of improvement picture of what is happening.
"Understanding the atomic detail of how they function gives us intimations to why these medications may impact one a player in the body that we need, however may likewise impact some portion of the body that we would prefer not to impact," she said.
"Understanding this can ideally educate future researchers dealing with tranquilize disclosure this is the means by which we think these sorts of medications work."
Dr. Buyan trusts the exploration will better advise researchers about the likelihood of changing the structure of existing medications, or in outlining another medication to ensure that it does what is expected without the symptoms.
Non - Toxic Cancer Drugs : New Insignt
Light-activated cancer drugs without toxic side effects : Most useful drugs for future
Future tumor tranquilizes that are actuated by light and don't cause the harmful symptoms of ebb and flow chemotherapy medicines are nearer to turning into a reality, on account of new research made conceivable by the Monash Warwick Alliance, an intercontinental joint effort between the University of Warwick (UK) and Monash University (Australia).
Driven by Robbin Vernooij, a joint PhD understudy from the Monash Warwick Alliance, crisp knowledge has been picked up into how a spearheading platinum-based chemotherapy sedate applicant - trans,trans,trans-[Pt(N3)2(OH)2(py)2] - capacities when actuated by light.
The treatment - initially created by Professor Peter Sadler's examination aggregate in the University of Warwick's Department of Chemistry - is an inorganic-metal compound with a bizarre system, which slaughters growth cells in particular focused on regions, with an end goal to limit lethal reactions on sound tissue.
Totally idle and non-harmful oblivious, the treatment can be embedded into carcinogenic zones, its capacities activated just when coordinated light hits it - making the compound corrupt into dynamic platinum and discharging ligand particles to assault disease cells.
Utilizing an old spectroscopic system - infrared spectroscopy - the scientists watched the end result for the structure of the compound by following the metal and in addition atoms discharged from the compound.
The specialists shone infrared light on the inorganic-metal compound in the research facility, and estimated the vibrations of its particles as it was initiated.
From this, they found the substance and physical properties of the intensify: a portion of the natural ligands, which are connected to the metal molecules of the compound, wind up confined and are supplanted with water while different ligands stay stable around the metal.
This new knowledge into the mechanics of the treatment offers new expectation that photoactive chemotherapy medicate hopefuls, for example, trans,trans,trans-[Pt(N3)2(OH)2(py)2], will advance from the research facility to future clinical trials.
Robbin Vernooij, lead creator and joint specialist from the Monash Warwick Alliance, remarked: "The present weaknesses of most chemotherapeutic operators are lamentably certain, and in this way there is continuous push to grow new treatments and enhance our comprehension of how these specialists function in push to create more powerful, as well as more specific, treatments to lessen the weight on patients.
"This is an energizing advance forward, exhibiting the energy of vibrational spectroscopic systems joined with present day processing to give new bits of knowledge on how this specific photoactive chemotherapeutic specialist functions, which conveys us one bit nearer to our objective of making more particular and powerful tumor medications."
Dwindle Sadler, Professor of Chemistry at the University of Warwick, remarked: "About portion of all chemotherapy medications for growth current utilize a platinum compound, however in the event that we can present new platinum aggravates that dodge symptoms and are dynamic against safe tumors, that would be a noteworthy progress.
"Photoactivated platinum mixes offer such potential outcomes. They don't murder cells until illuminated with light, and the light can be coordinated to the tumor so staying away from undesirable harm to typical tissue.
"It is vital that we see how these new light-actuated platinum mixes execute growth cells. We trust they assault tumor cells in absolutely new ways and can battle protection. Understanding at the sub-atomic levels requires utilization of all the propelled innovation that we can marshal. For this situation, progresses have been made conceivable by an exceedingly gifted research understudy working with cutting edge gear on inverse sides of the globe.
"We trust that new methodologies including the blend of light and chemotherapy can assume a part in combatting the present weaknesses of malignancy treatment and help to spare lives."
The larger part of tumor patients who experience chemotherapy treatment as of now get a platinum-based compound, for example, cisplatin. These treatments were created over 50 years back, and cause lethal reactions in patients, assaulting sound cells and in addition harmful ones.
There is additionally a developing protection from more conventional malignancy treatments, so new medications are urgently required.
The exploration was done between six research bunches at both the University of Warwick and Monash University, and was made conceivable through the universally famous shared mastery and assets over the Monash Warwick Alliance.
New use of existing drug in preventing type 1 Diabetes
Existing medication successful at forestalling beginning of Type 1 diabetes in 60% of patients
A medication regularly used to control hypertension may likewise help keep the beginning of sort 1 diabetes in up to 60 percent of those in danger for the sickness, as indicated by analysts at the College of Colorado Anschutz Therapeutic Grounds and the College of Florida in Gainesville.
"This is the primary customized treatment for type 1 diabetes anticipation," said Aaron Michels, MD, an analyst at the Barbara Davis Place for Youth Diabetes and partner teacher of drug at CU Anschutz. "We made this revelation utilizing a supercomputer, on the lab seat, in mice and in people."
The medication, methyldopa, has been utilized for more than 50 years to treat hypertension in pregnant ladies and youngsters. It is on the World Wellbeing Association's rundown of fundamental medications.
Be that as it may, in the same way as other medications utilized for one condition, Michels and his partners thought that it was helpful for something absolutely inconsequential.
Exactly 60 percent of individuals in danger of getting Type 1 diabetes have the DQ8 particle which altogether builds the possibility of getting the illness. The analysts trusted that on the off chance that they could piece particularly the DQ8 atom they could likewise hinder the beginning of the illness.
"All medications have off-target impacts. On the off chance that you take excessively acetaminophen you can hurt your liver," Michels said. "We took each FDA endorsed little particle medicate and broke down HLA-DQ8 official through a supercomputer. We hunt a thousand introductions down each medication to recognize those that would fit inside the DQ8 particle restricting notch."
In the wake of running a huge number of medications through the supercomputer, they found that methyldopa blocked DQ8, as well as it didn't hurt the safe capacity of different cells like numerous immunosuppressant drugs do.
The examination crossed 10 years and its adequacy was appeared in mice and in 20 compose 1 diabetes patients who participated in a clinical trial at the Barbara Davis Community for Youth Diabetes at the College of Colorado Institute of Pharmaceutical.
"We would now be able to foresee with right around 100 percent exactness who is probably going to get compose 1 diabetes," Michels said. "The objective with this medication is to postpone or keep the beginning of the infection among those in danger."
Recent Clinical Trial Revealed Cure for Bronchiectasis
Chinese researchers report first trial supported autologous respiratory organ somatic cell transplantation
Bronchiectasis is that the permanent injury to the respiratory organ that damages the cartilaginous tube structure of respiratory organ. this is often the chronic injury that lasts for lasting. For patients full of chronic respiratory organ diseases, respiratory organ somatic cell transplantation might be their biggest, if not last, hope.
Now for the primary time the technique of create the broken respiratory organs have acquire existence victimisation autologous lung somatic cell transplantation during a pilot trial.
In 2015 author and his colleagues known p63+/Krt5+ adult stem cells during a mouse respiratory organ, that had potential to regenerate respiratory organ structures together with bronchioles and alveoli Tongji University and Kiangnan somatic cell Institute is specializing in respiratory organ stem cells in humans instead of mice.
The researchers found that a population of basal cells labelled with associate degree SOX9+ marker had the potential to function respiratory organ stem cells in humans used respiratory organ bronchoscopy to push aside and amplify these respiratory organ stem cells from little samples. About 0.2% of the cells from every brush were respiratory organ stem cells. The genetic stability and molecular phenotypes of those cells might be well maintained over scaled growth
To check the capability of respiratory organ stem cells to regenerate respiratory organ tissue in vivo, the team transplanted the GFP-labelled human respiratory organ stem cells into broken lungs of immunodeficient mice.
Further histologic analysis showed that somatic cell transplantation with success regenerated human cartilaginous tube and alveolar structures within the lungs of mice. additional significantly, the host capillaries rose round the regenerated human alveoli structures, that indicated the formation of practical metabolic process units as incontestable by the gold nanoparticle trailing technique. Also, the fibrotic space within the dislocated lungs of the mice was replaced by new human alveoli once receiving somatic cell transplantation. blood gas analysis showed that the respiratory organ perform of the mice was considerably recovered.
One year once transplantation, 2 patients delineate relief of multiple metabolic process symptoms like coughing and dyspnoea. CT imaging showed regional recovery of the expanded structure. Patient respiratory organ perform began to recover 3 months once transplantation, that maintained for one year.
The respiratory organ somatic cell trial in China has been licenced by CFDA and National Health and birth prevention Commission. A multi-centre, placebo-controlled study is being distributed. Up to now, the team has performed eighty somatic cell transplantation cases in total, involving completely different classes of metabolic process diseases together with bronchiectasis, chronic clogging respiratory organ wellness and opening respiratory organ wellness.
Recent Revealatons in Drug Trial Protocol: BABE 2018
Medicate trial protocol redactions by industry sponsors Revealed
Modern research distributed by the Journal of the Royal Society of Medicine uncovered the degree of redactions in conventions for industry-sponsored randomized medicate trials. Trial conventions are required for an appropriate evaluation of the veracity of drug trial reports. The analysts, from the Nordic Cochrane Middle in Copenhagen, found broad redactions in the conventions for commercially supported trials they gotten from investigate morals committees in Denmark. The ponder is accepted to be the to begin with orderly evaluation of which data in trial conventions pharmaceutical companies do not wish to reveal to independent analysts.
Executive of the Nordic Cochrane Centre, said: "We wished to compare the data in the protocols with the data given to the patients in order to assess whether the trials were moral and essential and whether basic data around the benefits and the harms of the drugs had been covered up from the patients."
It is troublesome to get access to drug trial conventions so Executive of the Nordic Cochrane Centre and other researchers utilized the Danish Freedom of Information Act to request access to 78 trial conventions affirmed by a inquire about morals committee from October 2012 to Walk 2013. Eight conventions were prohibited since they did not meet the inquire about incorporation criteria. Only 17 of 34 conventions for commercially supported trials were unredacted, compared to 34 of 36 non-commercially supported trials.
The redactions were most broad in those areas of the protocol where there is experimental proof of significant issues with the dependability of distributed drug trials. These incorporate the definition of persistent results, the discovery and examination of antagonistic occasions and the sponsor's get to to approaching data while the study is running.
Executive of the Nordic Cochrane Centre, said: “The sum of redactions in the protocols we gotten was so tremendous that it made them rather useless for surveying the moral defense for the ponders and to recognize disparities with ensuing publications. We could not recognize any genuine basis for the redactions. The current mistrust in industry-sponsored drug trials can only alter in case the industry offers unrestricted access to its trial conventions and other significant archives and information."
Advances in Clinical Trial Management : BABE 2018
Clinical Trials : Rigid draft rules put new responsibilities for Drug Makers
Pharmaceutical firms conducting clinical trials of drugs in India will no longer be able to avoid duty in case of harm or passing of participants.
According to modern draft rules for clinical trials and new drugs, in case the sponsor comes up short to supply “medical management” to their trials, not only will the trial be cancelled, but the company will moreover be restricted from holding any more trials.
However, in a boost to firms needing to conduct trials on drugs proposed to be made and showcased in India, the consent for trials will be allowed inside 45 days.
Moreover, in case the trial subject endures from any other sickness amid the clinical trial or BA/BE study, the support ought to give necessary therapeutic administration and auxiliary care. “Where the support or the individual who has obtained consent from the central authorizing specialist fails to supply restorative administration, the authorizing specialist might, after managing an opportunity of being listened, suspend or cancel the clinical trial or BA/BE studies or limit the support to conduct any encourage clinical trial,” says the proposed administrative system for clinical trials and modern drugs. Mint has surveyed a duplicate of the last draft.
According to the draft new drugs and clinical trials rules, 2017, companies will have to pay remuneration if the drug fails to supply the planning helpful impact or where the required standard care or protect medicine, although accessible, was not given to the subject.
As per the modern draft rules, if the company proposes to conduct the clinical trial of a modern drug or an investigational modern medicate and moreover the new medicate is proposed to be made and showcased in India, not only the authorization will be allowed in a time bound way of 45 days, in case the central permitting specialist fails to communicate, the “permission to conduct the clinical trial should be regarded to have been granted”.
BABE 2017
8th World Congress on Bioavailability & Bioequivalence: Pharmaceutical R & D Summit hosted by the Conference Series was held during June 26-27, 2017 at San Diego, California, USA at Hilton San Diego Mission Valley with the theme “Throwing Light on Latest Techniques for Optimization of Drug Solubility, Delivery and Stability for Maximization of Product Life Cycles”, which got magnificent response. With the support and guidance of Organizing Committee Members, Editorial Board Members and astonishing presentations of all participants this prominent summit became more impressive.
We received active participation from various scientists, researchers, students and leaders from the field of Pharmaceuticals who made this event successful.
Conference Series would like to convey a great appreciation to Dr. Palmer Taylor & Dr. Pradeep Kakarla as Keynote speakers and Dr. Sudheendra K. for moderating the event.
Keynote Speaker supported this event with sustainable excitement for grand success of this prominent conference. We are expecting to meet the attendees once again at another part of the world for some other pharmaceutical conference.
BABE 2016
Our Cordial thanks to all of our wondrous and famed speakers, conference attendees, students, associations and guests for shaping BABE 2016 an ineradicable event and a mile stone.
Conference Series hosted the 7th World Congress on Bioavailability and Bioequivalence: BA/BE Studies Summit during August 29-31, 2016 at Atlanta, USA. The conference was designed around the theme of “Amalgamation of traditional techniques to advanced studies which redefine BA/BE approach and perception” and was a great success where eminent keynote speakers from various reputed companies made their resplendent presence and addressed the gathering. Moreover, the networking sessions laid the foundation for some time worthy collaborations between many start-up and big industries. The post conference networking lunch session witnessed a number of B2B meetings that are turning up to be mutually beneficial to both the organizations who had gone in for the business meetings.
BABE 2016 witnessed an amalgamation of peerless speakers who enlightened the crowd with their knowledge and confabulated on various new-fangled topics related to the field of Bioavailability and Bioequivalence. This congress not only brought forward the latest developments in the field but also provided solutions to the numerous challenges encountered in generic drug sector.
Conference Series would like to convey a warm gratitude to all the Honourable guests, Keynote Speakers, Delegates, Media Partners and Exhibitors for their participation in BABE 2016. The conference was initiated with a series of lectures delivered by both Honorable Guests and members of the Keynote forum. The list includes:
· Jim Jingjun Huang, Ascendia Pharmaceuticals, USA
· Mewa Singh, Meda Biotech LLC, USA
· Akwete Lex Adjei, Rhodes Pharmaceuticals L.P, USA
We on behalf of the conference specially thank Kateryna Zupanets for her support as a moderator for the conference. We also thank Sue Duran, Auburn University, USA and Muneesh Garg, Sitec Labs, India for their support for success of the conference. Conference Series also took the privilege of felicitating BABE 2016 Organizing Committee, Keynote Speakers, and Chair and Co-Chairs whose support made conference a great success with various sessions and multiple presentations.
Past Reports Proceedings Gallery
BABE-2015
BABE-2015 which was scheduled during August 17-19, 2015 at Chicago, USA with the theme “Advance Approaches in Discussion of Current Issues & Future Possibilities in Bioavailability and Bioequivalence Studies”. The conference had several workshops, multiple sessions, Keynote presentations, panel discussions and Poster sessions. We received active participation from various leaders from the fields of Bioavailability and Bioequivalence Research Centres, Professors, Companies, Researchers, students and Research Professionals, who made this event a Grand Success.
Various sessions were carried out with multiple presentations from speakers all around the world. Major Sessions to be discussed were:
· Emerging Bioavailability and Bioequivalence Studies
· Bioanalytival Methodology
· Contempory challenges of drugdesign ,discovery and development
· Regulatory policies procedures prerequisites for clinical research
· Managing BA/BE studies
· Relevance of Genetics to BA/BE in Drug Development
· Bioavailability, Bioequivalence and Drug Product Selection
· Advances in Assessment of Bioequivalence
· Need for conducting BA/BE studies
· Metabolic Pathways and Changes in Nutrient Bioavailability
· Clinical Pharmacology and Therapeutics
· Study Designs
· Factors Affecting Bioavailability
The conference was initiated with a series of lectures delivered by both Honorable Guests and members of the Keynote forum. The list includes:
- Paramjeet Kaur, U.S. Food and Drug Administration, USA
- Jim Jingjun Huang, Ascendia Pharmaceuticals, USA
- Keith Gallicano, Novum Pharmaceutical Research Services, USA
Series of workshops includes expert presentation by Paramjeet Kaur, U.S. Food and Drug Administration, USA, on “Biowaiver approaches for generic drug products in the US: case studies”, Jim Jingjun Huang, Ascendia Pharmaceuticals, USA on “Rational design of amorphous solid dispersion for solubility and bioavailability enhancement”, Keith Gallicano, Novum Pharmaceutical Research Services, USA on “Bioequivalence of topical corticosteroids: Design and data analysis challenges with the vasoconstrictor assay”. We specially thank Murat Sari, Pharmactive, Turkey, for his support as a moderator for the conference. We also thank Subrata Deb, Roosevelt University College of Pharmacy, USA for his support for success of the conference.
OMICS International offers its heartfelt appreciation to the Organizing Committee Members, namely Harish Pant, G. Ali Mansoori, A. M. Abd El-Aty, Sachin S Devi who supported us throughout the event. Also, it would be incomplete without thanking all our expert presenters from all around the world which includes various outside experts, company representatives and other eminent personalities who supported the conference by facilitating the discussion forums.
With the grand success of BABE-2015, OMICS International is proud to announce the “7th World Congress on Bioavailability & Bioequivalence: BA/BE Studies Summit " to be held during August 29-31, 2016 at Atlanta, USA.
Related Universities
Related Hospitals
Conference Highlights
- Drug Design and development: Challenges
- Bioavailability Studies and Assessment
- Drug Metabolism
- Bioequivalence Studies and Assessment
- Pharmacology- PK & PD approach
- Pharmaceutical Formulations
- Clinical Research Vs Clinical Trails
- BCS & IVIVC Based Biowaivers
- Recent approaches to Biosimilars
- Drug Safety: Pharmacovigilance Scope
- Contract Research Organizations
- Regulatory Requirements and Approaches
- Pharmaceutical Industry: Entrepreneur Meet
- Recent advancements in BA/BE Research
- Bioavailability and Bioequivalence
- Biowaivers: Criteria
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | April 16-18, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | Day 3 |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Bioequivalence & Bioavailability
- Journal of Bioanalysis & Biomedicine
- Pharmaceutica Analytica Acta
Abstracts will be provided with Digital Object Identifier by